Sulfur is an essential element for all life, and is widely used in biochemical processes. In metabolic reactions, sulfur compounds serve as both fuels and respiratory (oxygen-replacing) materials for simple organisms. Sulfur in organic form is present in the vitamins biotin and thiamine, the latter being named for the Greek word for sulfur. Sulfur is an important part of many enzymes and in antioxidant molecules like glutathione and thioredoxin.
Organically bonded sulfur is a component of all proteins, such as the amino acids cysteine and methionine. Disulfide bonds are largely responsible for the strength and shape of proteins. Since sulfur bonds are required for proteins to maintain their shape, and these bonds determine the biological activity of the proteins, we can see why sulfur is critical for health and life itself. There is no doubt that sulfur helps us battle cancer so it’s a good time to become more familiar with this basic element.
Sulfur is required for the proper structure and biological activity of enzymes. If you don’t have sufficient amounts of sulfur in your body, the enzymes cannot function properly. This can cascade into a number of health problems since, without biologically-active enzymes, your metabolic processes cannot function properly.
Sulfur enables the transport of oxygen across cell membranes.
Because sulfur is directly below oxygen in the periodic table, these
elements have similar electron configurations. Sulfur forms many
compounds that are analogs of oxygen compounds and it has a unique
action on body tissues. It decreases the pressure inside the cell. In
removing fluids and toxins, sulfur affects the cell membrane. Sulfur is
present in all cells and forms sulfate compounds with sodium, potassium,
magnesium, and selenium. Organic sulfur, in addition to eliminating
heavy metals, regenerates, repairs and rebuilds all the cells in the
body.Mercury & Sulfur
Sulfur is very complicated topic because:
Thiol poisons, especially mercury and its
compounds, reacting with SH groups of proteins, lead to the lowered
activity of various enzymes containing sulfhydryl groups. This produces a
series of disruptions in the functional activity of many organs and
tissues of the organism. - Professor I. M. Trakhtenberg[2] - Russia
Mercury, in its various forms, has a great attraction to the sulfhydryls or thiols—these sulfa bonds. A thiol is any organic compound containing a univalent radical called a sulfhydryl and identified by the symbol -SH (sulfur-hydrogen).Enzymes are proteins, and like all proteins they consist of chains of amino acids. These chains have to be faulted in a specific way to give the enzyme its activity. The structure of many enzymes is ensured by cross-bonding of the amino-acid chains. These cross-bonds consist of double sulfur bonds. Sulfur bridges are covalent S-S bonds between two cysteine amino acids, which tend to be quite strong. These sulfur bonds are damaged when poisonous substances that are not naturally present are added to the cellular and blood environments. Mercury binds to the -SH (sulfhydryl) groups, resulting in inactivation of sulfur and blocking of enzyme functions while producing sulfur metabolites with high toxicity that the body has difficulty handling. Sulfur is essential in enzymes, hormones, nerve tissue and red blood cells. These sulfur bonds are crucial to human biology.
If the geometry of insulin has been changed by
mercury, the message that insulin has arrived to give glucose to the
cell is not received.
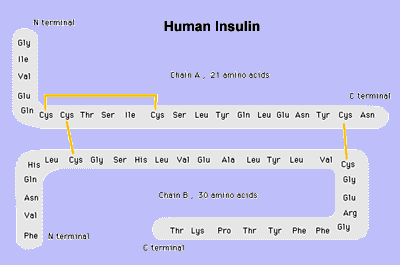
Amino Acid Structure of InsulinYellow lines indicate disulfide bonds.
Various molecules or atoms will affect the rate of an
enzyme-catalyzed reaction by binding to the enzyme. Some bind at the
same site as the substrate (the active site) and prevent the substrate
from binding. Others bind at sites on the enzyme remote from the active
site and affect activity by modifying the shape of the enzyme. Many of
these molecules reduce the activity of the enzyme and are referred to as
inhibitors.Mercury is the most potent enzyme inhibitor that exists; it is in a class of its own and well deserves its title as the most toxic non-radioactive element. Since mercury and lead attach themselves at these highly vulnerable junctures of proteins, they can readily provoke biochemical shifts and then morphological changes in the body. Transsulfuration pathways in the body are fundamental for life. When mercury blocks thiol groups, cellular proteins lose their reactive properties and lose their ability to carry out their routine function.
Because glycemic regulation is one of the body’s
most central homeostatic mechanisms, mercury’s attack is most
problematic, even at low concentrations, and indicates that it is
playing a great role in the dramatic rise of diabetes.
Insulin has three sulfur-containing cross-linkages and the insulin receptor has a tyrosine-kinase-containing sulfur bond; these are the preferred targets for binding by both mercury and lead. Should
mercury attach to one of these three sulfur bonds, it will interfere
with the normal biological function of the insulin molecule.
The average adult inhales thousands of trillions of mercury atoms a day
from a mouthful of amalgams; fish provides trillions more, the air
more, and in children, vaccines provide surges of trillions of mercury
molecules per day in the form of ethyl-mercury, which is vastly more
toxic than metallic mercury. Insulin molecules are directly assaulted as
are insulin receptor sites.We are all receiving, just through our air, water and food, about a microgram of mercury a day. Sounds like very little until you calculate that a microgram contains 3,000 trillion atoms each of which hold the potential to deactivate insulin and the receptor sites crucial to their function. Then you have to add the amount leaking from each of your dental amalgams, the mercury injected with your flu shot, and your proximity to a coal-fired plant or other mercury-contaminating source point like crematoriums and municipal incinerators.
Sulfur is present in all proteins, which makes it universally available throughout the body for binding with mercury. Some of the important biochemical sulfur-containing compounds of the body besides insulin are glutathione, prolactin, growth hormone, and vasopressin.
The bottom line is that no other element including oxygen has more of an ability to combine with other elements than sulfur. All the metals except gold and platinum combine with sulfur to form inorganic sulfides. Sulfur combines with aluminum to form aluminum sulfate, it combines with barium to form barium sulfate, and it combines with strontium to form strontium sulfate.
If you not getting the hint, I will say it outright! Sulfur is crucial for detoxification and chelation of heavy metals and radioactive particles, which behave like heavy metals chemically.
Reference:
http://drsircus.com/medicine/cancer/cancer-sulfur-garlic-glutathione
Other information:
www.cellularoxygenation.com
Not all Sulfur is the same. There are many people and places that sell it, but only a very few who sell the beneficial 99.9% crystal sulfur. At www.Cellular-Oxygenation.com we get it from the testing lab to assure excellent quality. Do not be fooled into thinking you can buy a cheaper version and get the same results.
No comments:
Post a Comment